The DHE Twinning “Strengthening the Capacity for the Analysis of Electronic Healthcare Data” (SCAN) has proved to be a successful example of the project addressing better data to advance research, disease prevention and personalised health and care.
The Digital Health Europe (DHE) twinning SCAN enabled knowledge transfer between two partners – the UK Medicines and Healthcare Products Regulatory Agency (MHRA) as an originator and the Croatian Agency for medicinal products and medical devices (HALMED) as an adopter. MHRA’s extensive experience in the use of electronic healthcare data for monitoring medicine safety was used to set up a similar process within HALMED’s pharmacovigilance department. Thanks to the DHE project, HALMED managed to establish a sustainable system for using the existing Croatian Central Health Information System (CEZIH) for observational research, generation of scientific evidence on drug-related issues and post-marketing drug regulatory decision-making. This has enabled researchers to use and analyse data sources, offering scope far beyond traditional data collection methods: with bigger size and population coverage, faster speed of access, the richness of data and the possibility for easier linking of information from different sources.
To implement the project successfully, partners first analysed each other’s status concerning electronic healthcare data analysis. During this step, they worked on providing an overview of all aspects of the MHRA process for electronic healthcare data analysis and interpretation, while also performing a gap analysis of the HALMED system to help streamline further development of the process within HALMED.
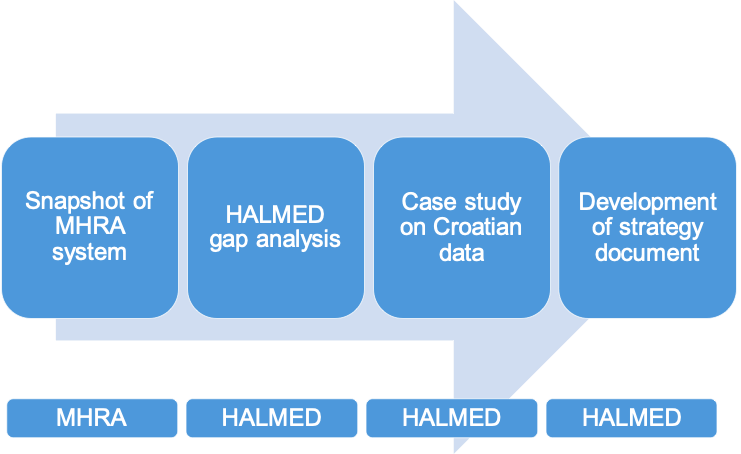
Next, HALMED developed a case study concept on the data from Croatian Central Health Information System. Under the supervision of MHRA, the Croatian agency simulated and tested the process steps with real data.
As a final step of the SCAN project, researchers from both agencies developed a strategy document for the inclusion of electronic healthcare data analysis into HALMED practice. In total, 7 experts (4 from HALMED and 3 from MHRA) contributed to the consistent and smooth implementation of the twinning.
In light of the implementation of the digital solution and the transfer of the expertise from the originator, through the twinning the Croatian Agency for medicinal products and medical devices is now not only able to collaborate with experts with extensive real-life experience in the field, but to share some of the electronic data analysis findings with Croatian government institutions and collaborative bodies, such as the Croatian Institute of Public Health (HZJZ) and the Croatian Health Insurance Fund (HZZO). This way, the results of the SCAN twinning can be used to help address public health issues, improve health technology assessment, pricing, and reimbursement.
